Background: Matched unrelated donors (MUD) have become an acceptable alternative donor for patients requiring potentially curative hematopoietic stem cell transplantation (HSCT) and lacking a matched sibling donor. Patients may undergo a bone marrow (BM) or peripheral blood stem cell (PBSC) graft, with previous studies showing similar survival outcomes. However, BM grafts have shown a lower incidence of chronic graft-versus-host disease (GVHD) but a higher relapse rate and slower engraftment. We aimed to compare the outcomes of BM versus PBSC in patients undergoing MUD HSCT.
Methods: For this retrospective single-center study, we included all (n=276) adult MUD HSCT recipients at the University of Kansas Medical Center who had at least one year of follow-up (August 2016 to July 2021). We conducted a bivariate analysis using chi-squared and ANOVA tests. The Kaplan-Meier and regression analyses were also performed. Data analyses were conducted using SPSS version 28. Statistical significance was considered at p<0.05.
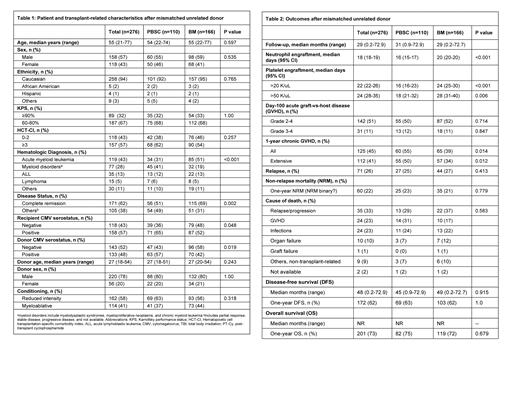
Results: Our study included 276 MUD patients including 110 PBSC (40%) and 166 BM (60%) grafts. The median age was 55 (range 21-77) years, and 158 patients (57%) were males. Eighteen patients (6%) were ethnic minorities, and 89 (32%) patients had a Karnofsky performance status of ≥90%. 118 (43%) patients had a hematopoietic cell transplantation-specific comorbidity index of 3 or higher. Hematologic diseases included acute myeloid leukemia (n=119, 43%), myeloid disorders (n=77, 28%), acute lymphoblastic leukemia (ALL) (n=35, 13%), Lymphoma (n=15, 5%), and others (n=30, 11%). Sixty-two percent of patients were in complete remission at the time of transplantation, and patients not in remission included myelodysplastic syndrome, myelofibrosis, and lymphoma patients. Reduced-intensity conditioning (RIC, n=162, 58%) and myeloablative conditioning (MAC) (n=114, 41%) were used. The median follow-up was 55 (21-73) months. Overall survival (OS) was not reached in either BM or PBSC MUD HSCT group, while a median disease-free survival of 45 and 49 months was noted in PBSC and BM groups respectively (p=0.915). We observed similar rates of relapse (25% vs 27%, p=0.413), one-year overall survival (75% vs 72%, p=0.679), one-year disease-free survival (63% vs 62%, p=1.00), and one-year non-relapsed morality (NRM, 23% vs 21%, p=0.779) after PBSC or BM graft. Bone marrow graft recipients had delayed engraftment of neutrophils (20 days vs 16 days, p<0.001) and platelets (>20 K/uL: 24 days vs 16 days, p<0.001; >50 K/uL: 28 days vs 18 days, p=0.006) compared to PBSC recipients. Bone marrow graft recipients had similar rates of grade II-IV acute GVHD (50% vs 52%, p=0.714) and grade III-IV GVHD (12% vs 11%, p=0.847), but lower rates of 1-year chronic GVHD (39 % vs 55%, p=0.014) compared to PBSC recipients. In the subgroup analysis stratified by conditioning intensity, faster engraftment of neutrophils (median 16 vs 21 days, p<0.001) and platelets (>20 K/uL: 18 days vs 27 days, p<0.001; >50 K/uL: 18 days vs 33 days, p<0.001) was observed with PBSC as compared to BM graft in myeloablative transplant recipients, while there was no statistically significant association with the rates of acute or chronic GVHD, NRM, Relapse, DFS, and OS after a PBSC or BM graft. Among the RIC transplant recipients, faster neutrophil engraftment (mean 17 vs 20 days, p<0.001) and higher 1-year extensive chronic GVHD (52% vs 32%, p=0.015) were observed with PBSC compared to BM graft whereas no statistically significant difference was noted in platelet engraftment, acute GVHD, NRM, Relapse, DFS, and OS after a BM or PBSC graft.
Conclusions: Allogeneic matched unrelated donor hematopoietic stem cell transplant recipients had similar rates of overall and disease-free survival, relapse, non-relapse mortality, and acute GVHD after a bone marrow or peripheral blood stem cell graft; however, delayed neutrophil and platelet engraftment with a lower incidence of chronic GVHD was noted after a bone marrow graft compared to peripheral blood stem cell graft.
Disclosures
Ahmed:Kite: Consultancy, Research Funding; BMS: Consultancy. McGuirk:Pluristem Therapeutics: Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Astellas Pharma: Research Funding; Bellicum Pharmaceuticals: Research Funding; Gamida Cell: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal